Why Filters Cartridge Require Bubble Point Integrity Test and Its Importance
INTRODUCTION OF BUBBLE POINT TEST
The Bubble Point Test is one of the most widely used and reliable methods for verifying the integrity of membrane filter cartridges.
It allows engineers and quality managers to confirm whether a filter meets its rated pore size and remains free from damage before or after use.
In industries such as pharmaceuticals, food & beverage, microelectronics, and water treatment, even a minor filter defect can result in contamination, product loss, or regulatory non-compliance.
This is why understanding bubble point testing is essential for maintaining filtration safety and system performance.
In this guide, you will learn:
- ✔ The working principle of the bubble point test
- ✔ How to correctly perform a bubble point integrity test
- ✔ Typical bubble point values for different membrane materials
- ✔ How to interpret test results and avoid common errors
What is Bubble Point Filter Integrity Test?
The bubble point test is based on a fundamental physical principle:
the largest pore in a fully wetted membrane will allow gas to pass through first when pressure is increased.
During the test, the filter membrane is completely wetted with a compatible liquid.
Gas pressure is then gradually applied to one side of the filter.
As the pressure increases, the liquid held inside the membrane pores resists the gas flow due to surface tension.
When the applied pressure becomes high enough to overcome this surface tension in the largest pore,
a continuous stream of bubbles is observed at the downstream side.
This pressure point is defined as the bubble point.
Because the bubble point is directly related to the largest pore size, it serves as a reliable indicator of membrane integrity.
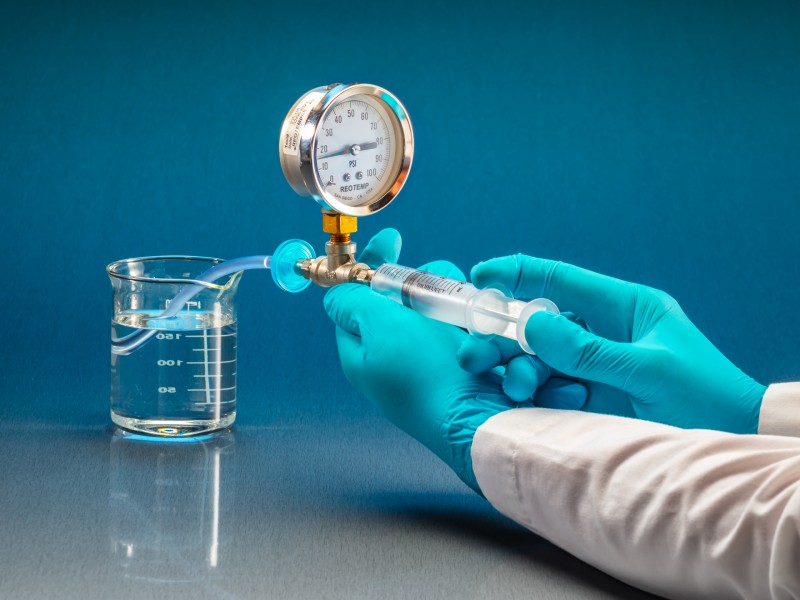
Bubble Point Test Procedure
To obtain accurate and repeatable results, the bubble point test must be performed following a standardized procedure:
- Pre-wet the filter cartridge
Ensure the membrane is completely wetted using a compatible liquid (e.g., purified water, IPA for PTFE membranes). - Install the filter in the test housing
Make sure all seals are properly tightened to avoid false leakage. - Gradually increase gas pressure
Apply clean compressed air or nitrogen at a controlled rate. - Observe bubble formation
Watch for a continuous and steady stream of bubbles, not isolated or random bubbles. - Record the bubble point pressure
Compare the measured value with the manufacturer’s specification.
Learn More >> Step-by-Step Procedure for Bubble Point Filter Integrity Test – Econe Filtration
Typical Bubble Point Values for Different Membrane Materials
| Membrane Material | Wettability | Bubble Point Characteristics | Common Applications |
|---|---|---|---|
| PES Filter | Excellent | Stable and consistent bubble point | Pharmaceutical, Food & Beverage |
| PVDF Filter | Moderate | Higher bubble point than PES | Water treatment, Chemicals |
| PTFE Filter | Poor (requires IPA) | Very high bubble point | Air and gas filtration |
| Nylon Filter | Good | Medium bubble point stability | Laboratory and general liquid filtration |
Learn More >> Bubble Point Test for Different Types of Filters
Econe Filtration offers a wide range of filter cartridges suitable for bubble point testing. The bubble point test is widely applied across different Econe Filtration filter cartridges to verify pore size integrity and filtration performance. The specific testing procedures may vary depending on the filter material and cartridge design.
1. PES Membrane Filter Cartridges
PES membranes are hydrophilic, making them easy to wet with water.
They typically show consistent bubble point values due to their uniform pore size distribution.
Ideal for applications in biopharmaceuticals and food & beverage, where precise microbial retention is required.
The bubble point test provides reliable verification of sterility assurance.
2. PVDF Membrane Filter Cartridges
PVDF membranes are naturally hydrophobic, often requiring alcohol-water pre-wetting before testing.
After proper wetting, the bubble point test confirms pore integrity for pharmaceutical and high-purity water systems.
PVDF membranes generally show higher bubble point pressures due to tighter pore structures and strong solvent compatibility.
3. PTFE Membrane Filter Cartridges
PTFE membranes are strongly hydrophobic, requiring wetting agents such as isopropyl alcohol (IPA) before testing.
Commonly used in air, gas, and aggressive chemical filtration.
The bubble point values are typically higher than hydrophilic membranes, ensuring excellent retention of fine particles and microorganisms.
Test results are especially critical in sterile vent filtration.
4. Nylon Membrane Filter Cartridges
Nylon membranes are naturally hydrophilic but have a higher affinity for water compared to PES.
Their bubble point values are stable and reproducible, making them suitable for pharmaceutical liquids, food, and laboratory-grade water.
Bubble point testing helps confirm performance in applications requiring low extractables and consistent flow rates.
5. Depth Filters (e.g., Melt-Blown or String-Wound Filter Cartridges)
Application: Pre-filtration in industrial and chemical processes.
Testing Method: Bubble point testing is less effective for depth filters because of their irregular pore structures. Instead, integrity is usually verified using challenge tests or alternative methods such as pressure hold
In summary, while membrane filters and pleated cartridges are most commonly validated using bubble point tests, hydrophobic and depth filters may require modified methods or alternative integrity testing techniques. Choosing the correct test method depends on filter design, material, and application.
Practical Example: Bubble Point Test for a 0.2 µm PES Filter Cartridge
For a typical 0.2 µm PES membrane filter cartridge used in pharmaceutical applications,
the minimum acceptable bubble point value is usually around 28 psi (1.9 bar), depending on the manufacturer.
If the measured bubble point is lower than the specified value, the possible causes may include:
- Incomplete membrane wetting
- Incorrect test pressure ramp rate
- Damaged membrane structure
- Instrument calibration errors
In such cases, it is recommended to re-wet the filter, verify instrument accuracy, and repeat the test before rejecting the cartridge.

Why Filter Cartridges Require Bubble Point Test
The bubble point filter integrity test is a non-destructive integrity test used to determine the pore size and structural soundness of a membrane filter or pleated filter cartridge. The principle is straightforward:
1. Verifying Structural Integrity
Filters can develop defects during:
Manufacturing – caused by uneven pore distribution or weak membrane bonding.
Transportation – due to mechanical shocks or improper packaging.
Installation and Use – from overtightening, improper handling, or exposure to aggressive chemicals.
Even small defects, such as pinholes, cracks, or sealing issues, can compromise filter integrity. Bubble point testing provides a simple yet effective way to detect such issues before filters are used in critical processes.
2. Ensuring Accurate Filtration Performance
A filter’s rated pore size is a promise that it will retain particles or microorganisms above a certain size threshold. If the actual pore size distribution deviates, the filter may fail to perform. Bubble point testing directly measures pore size consistency, ensuring that filters meet their stated specifications.
For example:
A 0.2-micron filter in pharmaceutical applications must be capable of retaining bacteria such as Brevundimonas diminuta. Bubble point testing verifies this retention ability before use.
A 0.45-micron filter used in beverage clarification must remove particles that affect taste and clarity. Bubble point testing confirms the filter will perform reliably.
3. Supporting Regulatory Compliance
Industries such as pharmaceuticals, biopharmaceuticals, and food processing are governed by strict international standards, including:
FDA (Food and Drug Administration) guidelines
GMP (Good Manufacturing Practices) requirements
ISO (International Organization for Standardization) certifications
These guidelines often mandate filter integrity testing to ensure sterility and product safety. Bubble point testing, as an internationally recognized method, allows companies to demonstrate compliance during audits and inspections.
4. Quality Assurance for Manufacturers and Users
For filter manufacturers, bubble point testing is part of routine quality control before shipping filters to customers. For end users, performing the test before and after use provides assurance that the filter has not been compromised during operation. This two-step approach—pre-use and post-use bubble point testing—ensures both system reliability and traceability.
5. Cost-Effective and Non-Destructive
Because bubble point testing does not damage the filter, it offers significant cost advantages. Unlike destructive methods (such as burst testing), filters remain usable after validation. This makes it ideal for industries that rely on high-value sterile filters and cannot afford waste.
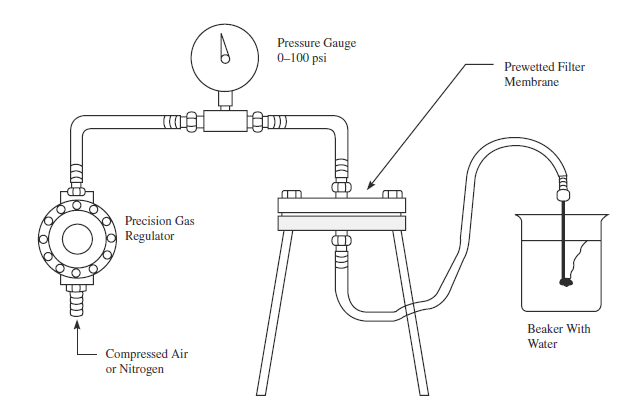
How Bubble Point Testing Is Performed
Manual Bubble Point Test
Traditionally, operators performed the bubble point test manually by immersing the downstream side of the filter in water and visually observing the formation of bubbles. While effective, this method depends on human judgment and may lack precision.
Automated Bubble Point Test
Modern industries now use automated integrity testing instruments. These devices apply controlled pressure, detect bubbles electronically, and record results with minimal human error. Automated systems:
Improve accuracy and repeatability
Provide data logging for regulatory audits
Reduce test time and labor costs
Industry Applications of Bubble Point Testing
1. Pharmaceutical and Biopharmaceutical Industry
Ensures sterile filtration for injectable drugs, vaccines, and biologics.
Demonstrates compliance with FDA and GMP standards.
Reduces contamination risks in life-critical products.
2. Food and Beverage Processing
Protects product flavor, aroma, and clarity.
Used in microbial stabilization of beer, wine, dairy, and bottled water.
Prevents spoilage by verifying filter performance before bottling.
3. Water Treatment and Ultrapure Water Systems
Confirms safety of municipal drinking water supplies.
Guarantees purity in ultrapure water used in pharmaceuticals and electronics.
4. Microelectronics and Semiconductor Fabrication
Prevents particles from contaminating microchips and circuits.
Ensures ultrapure water and chemicals remain free of contaminants.
Reduces product defects in high-tech industries.
5. Chemical Processing
Validates filter reliability in aggressive chemical environments.
Prevents costly downtime caused by contamination.
Ensures consistent product quality in sensitive processes.
Advantages of Bubble Point Testing
Accurate correlation between pore size and bubble point pressure
Non-destructive, allowing reuse of filters after testing
Internationally accepted for regulatory compliance
Quick and efficient, especially with automation
Applicable across multiple industries
Improves product safety and reduces risk of recalls
Bubble Point Testing vs. Other Integrity Tests
While bubble point testing is widely used, other filter integrity tests include:
Diffusion Test: Measures gas flow through wetted pores at pressures below the bubble point.
Pressure Hold (Decay) Test: Measures the rate of pressure drop over time in a pressurized system.
Water Intrusion Test: Commonly used for hydrophobic filters.
Compared to these, bubble point testing is faster and provides a direct relationship between pore size and test results, making it the preferred method in many industries.
Learn More >> How to Choosing the Right Method for Membrane Filter Integrity Test
Conclusion
The bubble point test is more than just a technical procedure—it is a cornerstone of filtration validation. By confirming pore size, detecting defects, ensuring regulatory compliance, and safeguarding against contamination, bubble point testing provides industries with confidence that their filters will perform reliably.
From pharmaceuticals and food safety to microelectronics and water purification, filters cannot be trusted without verification. That is why bubble point testing remains one of the most essential integrity tests in modern filtration science.
For industries where safety, purity, and reliability are non-negotiable, bubble point testing is not just important—it is indispensable.
References and Authoritative Sources
To enhance the article’s credibility and SEO authority, you can cite industry-recognized organizations and technical standards. Suggested references:
How to Process Bubble Point Testing for Hydrophobic Filters?
ASTM International – ASTM F316-03(2019): Standard Test Methods for Pore Size Characteristics of Membrane Filters by Bubble Point and Mean Flow Pore Test
U.S. Food and Drug Administration (FDA) – Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing — Current Good Manufacturing Practice
Pall Corporation – Filter Integrity Testing Technical Guide
Pall Integrity Testing Resources
MilliporeSigma (Merck KGaA) – Principles and Practices of Membrane Filter Integrity Testing
MilliporeSigma Integrity Testing Guide
- Understanding Bubble Point Test Filters: Principles and Applications





